Metabolomics is the study of the metabolome, i.e. all the metabolites present in a cell, tissue or organism at a given time. What are metabolites? Our body, in order to function, needs to destroy substrates and generate products during chemical reactions. In doing so, it needs co-factors, it releases some molecules and uses others. All these molecules necessary for intrinsic functions of our body, growth, development or reproduction, are metabolites.
How does metabolomics work?
The main technique used is nuclear magnetic resonance (NMR) and mass spectrometry (MS). These are biophysical techniques, based on the same principle, the study of the interaction between matter and light to which it will be exposed. However, the similarities stop there.
Nuclear magnetic resonance spectroscopy uses the physical characteristics of molecules. Indeed, each molecule is made up of atoms, themselves made up of an assembly of protons, neutrons and electrons. When the electrons position themselves around the nucleus of the atom, they fill what are called orbitals (a place where the probability of finding an electron is very high) in a precise order. This notion, of the order of quantum physics, is important to understand the existence of what are called spins: imagine that an orbital represents a box to fill, in each box, we can put two electrons with an opposite spin. When there is only one spin in this cell, it becomes mobilizable to make bonds with other atoms, in particular. In NMR, this is exactly what we do, we mobilize these free spins by passing a magnet over our sample to create a magnetic field. The sample is then irradiated with light and the way the molecules interact with the light will give us information about their structure. More precisely, we are interested in the spins of a specific atom (it would be too complicated to take them all into account), the proton (or H+), which has only one electron and only one spin to magnetize. Protons are present in all molecules and their environment, making them ideal targets: by irradiating a sample with light and recovering the proton spectrum, we obtain a very accurate image of the structure of molecules and their environment.
Mass spectrometry uses the physico-chemical properties of molecules. After an ionization step, where the molecules whose structure is to be determined are injected into the spectrometer and transformed into ions, it will separate the molecules. It bases its separation method on the ratio m/z, m being the mass of a molecule and z corresponding to the number of free orbitals (not occupied by a pair of electrons). Z is often considered as the “charge” of the molecule that is then noted q: calcium will not have a charge, but ionized calcium (Ca2+) will have 2. Once separated by their m/z ratio, the signal obtained by the separation of ions is transformed into electrical current and detected by a detector. Thus the spectrometer is able to identify molecules or protein fragments and will give its result in the form of a spectrum.
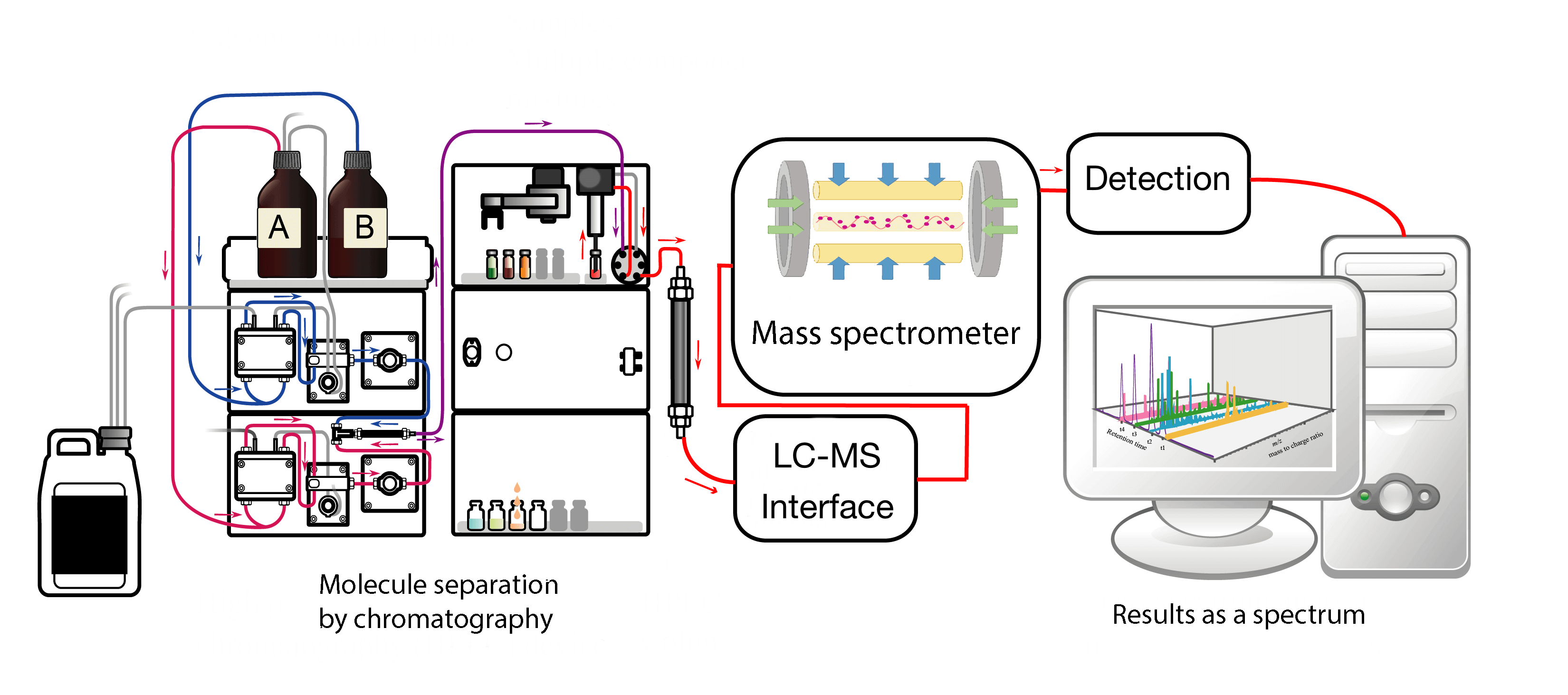
The data obtained with the different approaches to metabolomics are then analyzed using statistics. However, metabolomics generates large amounts of data and requires specific and complex bioinformatics tools. By using these techniques, several kinds of analyses can be performed:
- Targeted analysis, which allows the detection and quantification of known metabolites,
- Metabolic profiling, which studies a specific metabolic pathway by identifying, detecting and quantifying all metabolites involved,
- The metabolic footprint, allowing the comparison of metabolomes under different conditions (over time, after treatment, according to external pressures such as stress or heat…),
- The metabolomic approach, which allows the non-targeted identification and quantification of all metabolites present in a biological sample.
Metabolomics and aging
Several studies report an aging signature through metabolite analysis. One of them, in mice, interprets metabolomic results in urine and feces. Without going into the details of the extended names of the molecules identified, the team showed that there were changes in crucial signaling pathways, such as amino acid derivatives (important for protein synthesis), short-chain fatty acids (central in maintaining the intestinal microbiota), intermediates of choline and betaine (essential for cardiovascular health), nicotinamide and its derivatives (powerful actors for mitochondrial function) and ketone bodies (major modulators in liver health), among others[1]. At the same time, a team of researchers has highlighted the impact of caloric restriction on urinary metabolites and confirmed its beneficial effect on aging[2]. Another metabolomics study shows similar results, still in mice. For these, the analyses were not conducted on body fluids but directly in different tissues, showing a decrease in protein expression, and their respective transcripts, involved in phosphorylative oxidation, fatty acid oxidation, mitochondrial biogenesis, oxidative stress and apoptosis, all central phenomena in the aging process[3].
Beyond a global metabolomic signature, teams have also looked at specific changes. In the brain, a metabolic imbalance was identified, characterized by abnormal energy status (decreased NAD, increased AMP/ATP, accumulation of purin/pyrimidine) and altered nucleotide biosynthesis, accompanied by deregulation of oxidative phosphorylation. All these changes lead to a “metabolic drift” that prevents the brain from responding properly to external stimuli and compromises inter-neuronal communication[4]. In a Parkinson’s disease model, whose main alteration is linked to α-synuclein, a team demonstrated the deregulation of alanine and guanosine metabolism (both central to brain health) and acetyl-CoA oxidation and biosynthesis metabolism. This study confirms the importance of age on the onset of Parkinson’s disease and opens up new therapeutic and diagnostic avenues[5].
Metabolomics also has applications in other tissues and other diseases, such as cardiovascular diseases. In particular, a study showed that the use of metabolic profiling could identify biomarkers that predict major cardiovascular events (such as heart attacks, strokes or aneurysms) in the elderly, thus improving the follow-up and management of these patients[6].
Concerning the length of the telomeres, one of the major biomarkers of aging, an English team tested 280 metabolites in two cohorts of volunteers to determine their association with aging. Five metabolites have been identified as correlated with telomere length and functional measures of aging (blood pressure, cholesterol and pulmonary, hepatic and renal function). These five molecules belong to the metabolism of fatty acids and oxidative stress, suggesting that deregulation of these signaling pathways could be partly responsible for telomere reduction and aging[7].
Understanding the relationship between diseases, risk factors, biomarkers and aging are major objectives of medical research and more specifically, “omics” approaches. By combining these approaches, to obtain a systems biology, allowing a global understanding of the mechanisms of age and age-related diseases, it is possible to hope to find new solutions and to better understand the underlying complex mechanisms. Combined with epidemiology, metabolomics is therefore one of the most powerful tools at our disposal today to better fight against aging.
See all our articles on the “-omics” approaches
The “-omics” : a tool to better understand our aging process
What is behind the term “-omics? When we talk of genomics, transcriptomics or proteomics, what are we looking at? Here is a guide to explain it all!
Part 1: Understanding genomics for anti-aging research
How can we not mention genomics and how useful they are. It is the oldest “-omics” approach and still the most studied one even today. It gave birth to whole new concepts, such as epigenetics or systems biology, and opened up the scientific community to new horizons that we had never even dreamed ot!
Part 2: Transcriptomics, a constantly evolving science.

The discovery of non coding RNA led to a Nobel Prize. Enough to say it’s an important field of research! Transcriptomics is allowing the discovery of new tools, new mechanisms and led us to better understand the regulation of transcription.
Part 3: Proteomics, a mish-mash of research fields
 Above all, proteomics are a multidisciplinary approach, taking into account the interactions between fields, namely genomics and transcriptomics. It also refers to different concepts, from immunology to nutrition or cell function.
Above all, proteomics are a multidisciplinary approach, taking into account the interactions between fields, namely genomics and transcriptomics. It also refers to different concepts, from immunology to nutrition or cell function.
Part 4: Metabolomics, the last-born of the “omics”
 Last but not least! Metabolomics is a field that helps us understand very complicated regulation networks and the daily discovery of new cell-cell communication players.
Last but not least! Metabolomics is a field that helps us understand very complicated regulation networks and the daily discovery of new cell-cell communication players.
References:
[1] R Calvani, E Brasili et al., Fecal and urinary NMR-based metabolomics unveil an aging signature in mice, Experimental Gerontology, 2014; 49:5-11
[2] Zhang Y, Yan S, et al.Analysis of urinary metabolic profile in aging rats undergoing caloric restriction. Aging Clin Exp Res. 2012 Feb;24(1):79-84
[3] Houtkooper RH, Argmann C, Houten SM, et al. The metabolic footprint of aging in mice. Scientific Reports. 2011;1:134
[4] Ivanisevic J, Stauch KL, Petrascheck M, et al. Metabolic drift in the aging brain. Aging (Albany NY). 2016;8(5):1000-1013
[5] Chen X, Xie C, Sun L, Ding J, Cai H. Longitudinal Metabolomics Profiling of Parkinson’s Disease-Related α-Synuclein A53T Transgenic Mice. Singh PK, ed. PLoS ONE. 2015;10(8):e0136612
[6] S. Rizza,M. Copetti,C. Rossi et al., Metabolomics signature improves the prediction of cardiovascular events in elderly subjects, Atherosclerosis, 2014;232(2):260–264
[7] Zierer J, Kastenmüller G, Suhre K, et al. Metabolomics profiling reveals novel markers for leukocyte telomere length. Aging (Albany NY). 2016;8(1):77-86
Dr. Marion Tible

Author/Reviewer
Auteure/Relectrice
Marion Tible has a PhD in cellular biology and physiopathology. Formerly a researcher in thematics varying from cardiology to neurodegenerative diseases, she is now part of Long Long Life team and is involved in scientific writing and anti-aging research.
More about the Long Long Life team
Marion Tible est docteur en biologie cellulaire et physiopathologie. Ancienne chercheuse dans des thématiques oscillant de la cardiologie aux maladies neurodégénératives, elle est aujourd’hui impliquée au sein de Long Long Life pour la rédaction scientifique et la recherche contre le vieillissement.
En savoir plus sur l’équipe de Long Long Life
Dr Guilhem Velvé Casquillas

Author/Reviewer
Auteur/Relecteur
Physics PhD, CEO NBIC Valley, CEO Long Long Life, CEO Elvesys Microfluidic Innovation Center
More about the Long Long Life team
Docteur en physique, CEO NBIC Valley, CEO Long Long Life, CEO Elvesys Microfluidic Innovation Center
En savoir plus sur l’équipe de Long Long Life