Aging cancer itself
Cancer is a disease commonly associated with aging. Paradoxically, unlike most cells in our body, cancer cells do not age. This aging process of our cells, leading to their death, allows the body to get rid of the cells after a certain time. Indeed, an old cell may have accumulated damage over time, becoming deficient or even dangerous. This is the case for cancer cells, which are insensitive to this aging mechanism.
What if we could prevent these cells from remaining “young” forever? A group of 52 researchers, coordinated by Anne K. Voss and Tim Thomas, could have done that. In their article in Nature[1] they detail how they developed a molecule capable of aging lymphoma cells, a form of blood cell cancer.
KAT6A & KAT6B: key to lymphoma youth
Because cell aging is a normal phenomenon, rooted in their DNA, cancer must arm itself with tools to resist it. Two of them are the proteins KAT6A and KAT6B, belonging to the family of histone acetyltransferases. Their role is to modulate DNA activity. It has been shown before that many lymphomas over-express these proteins. In addition, mutated mice for which these proteins are less active can resist lymphomas almost 4 times longer. The researchers therefore decide to develop a molecule capable, without genetic modification, of blocking the action of these two proteins.
Nevertheless, it is a difficult challenge. They must find a molecule that only binds to these two proteins, without interfering with the others. This is not all, the fixed molecule can then have very different effects on the activity of the targeted proteins. Thus, it could have no effect, or even worse, make KAT6A and KAT6B even more active.
After a long optimization work, they manage to develop it. Moreover, when it is used to treat cancer cells in culture, they enter senescence and then stop any form of proliferation. In other words, exposed to this molecule, cancer cells age.
Aging cancer cells within the body
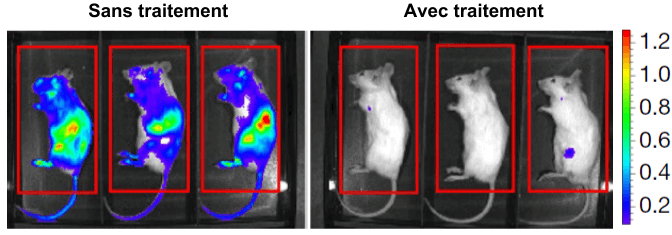
Treatment therefore has the desired effect on cancer cells in culture. But how do you make sure that the treatment is effective in fighting a real cancer? To do this, researchers use lymphoma cells that they inject directly into mice. These cancer cells have a particularity: they emit light through a bioluminescence process. This allows scientists to observe the tumor’s evolution inside the mouse itself. They then compare how lymphoma progresses depending on whether the mouse receives treatment or not.
The results are striking, as can be seen in the figure opposite, mice receiving treatment are able to control tumour expansion, unlike untreated mice. Moreover, additional tests show that the spleen of treated mice, one of the main sites of lymphoma, is almost devoid of cancer cells.
Thus, it would be possible to remind tumours that time passes and that they too are aging. By preventing it from proliferating, an essential step in its development, this new molecule acts as a real barrier against lymphoma. What if Anne K. Voss and Tim Thomas had just discovered tomorrow’s cancer treatment?
References:
[1] Jonathan B. Baell, David J. Leaver, …Tim Thomas. Inhibitors of histone acetyltransferases KAT6A/B induce senescence and arrest tumour growth. Nature (2018)
Baptiste Tesson

Author
Auteur
Baptiste is studying biology at the École Normale Supérieure de Lyon and bioengineering at the École Polytechnique Fédérale de Lausanne. He worked on the optimization of Cas9 as a tool for genome editing and on the emergence of blood stem cells in the zebrafish. He currently works on the patterning of the muscles, also in the zebrafish. He plans on doing a PhD in developmental biology.
More about the Long Long Life team
Baptiste étudie la biologie à l’École Normale Supérieure de Lyon et la bioingénierie à l’École Polytechnique Fédérale de Lausanne. Il a travaillé sur l’optimisation de la protéine Cas9 comme outil de modification de génomes et sur le développement des cellules souches du sang chez le poisson zèbre et travaille actuellement sur la mise en place des muscles chez le même animal. Il projette de réaliser un doctorat axé vers le développement animal.
En savoir plus sur l’équipe de Long Long Life

![[Video] Eurosymposium on Healthy Ageing, Brussels, 2018 Eurosymposium on Healthy Aging](http://www.longlonglife.org/wp-content/uploads/2019/07/P1310252-218x150.jpg)