Telomeres: at the heart of the aging process
What are telomeres?
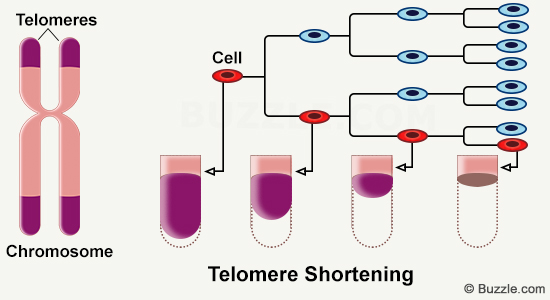
In the nucleus of each of the billions of cells that compose our bodies, chromosomes make up DNA. At the end of every chromosome, you can find small structures called telomeres. They progressively get shorter with time, and their length can be linked to age.
Telomeres are ribonucleoprotein complexes found at the extremities of chromosomes. They correspond to tandem repetitions of nucleotide sequences (TTTAGG) that shrink with each cellular replication. Throughout life, cells multiply. They accumulate cycles of division and replication, in order to renew damaged cells and tissue. In an aged organism, that is when telomere shortening occurs [1]. In this article, we will be looking at the way telomeres work and at the impact of their shortening on the body as a whole.
![]()
Aging, and the role of telomeres
Telomere shortening is directly linked to cell division. Because DNA polymerase cannot replicate the linear end of chromosomes, with DNA each replication cycle, there is a loss of some genetic material [1]. Since telomeres don’t contain coding sequences, no genetic information is lost. Therefore, telomeres are implicated in genome integrity preservation processes and are indispensable to cellular function.
When no mechanism intervenes to regenerate telomeres, and since telomere shortening occurs with each cell replication cycle, it indicates that cells cannot live indefinitely. The Hayflick limit is the maximum number of cell divisions that a cell can undergo [2]. It allows to relate telomere length to the lifespan of the cell.
![]()
Telomeres can trigger senescence, which is a cause of aging
The shorter telomeres become, the bigger the risk to lose some genomic information during the next cell division, inducing major cell dysfunction. That is why, with somatic cells, when telomeres reach the “critical” Hayflick limit, response pathways are activated in order to fix potential DNA damage. When these pathways are activated, the cell cycle is stopped, which can lead to cell senescence or death [3].
Throughout the aging process, cells accumulate multiple mitotic divisions which makes the risk of developping genetic anomalies rise. Telomeres then prevent the development of these damaged cells. Their measurement could give us information about the speed at which aging occurs, as well as about their biological age.
We call telomeres the “biological clockS” of our bodies.

![]()
Telomeres against tumor cells
Tumor cells are somatic cells that have malfunctioned as they develop and multiply very quickly. Their proliferation triggers a great number of cell divisions and consequently accelerates telomere shortening. The telomeres in these cells then quickly reach the Hayflick limit, which triggers the process previously described. This stops the cell cycle and sends the tumor cell into senescence. Cell proliferation is then stopped.
Telomere shortening occurs in the processes that prevent tumor cells from proliferating [3]. This normally allows to eliminate tumors before they become malignant and cancerous.
![]()
Telomeres and stem cells: aging essentials
There are mechanisms to maintain telomere structure that allow to prolong the life of some cells. Among them is the telomerase. This enzyme helps to synthesize telomeres [2].
Among them is the telomerase. This enzyme helps to synthesize telomeres [2].
Telomerase is absent from most of the organism’s cells. It is active in stem cells such as HSC, NSC and ESC: hematopoietic stem cells, neural stem cells, and epidermal stem cells. These cells are mainly involved in tissue renewal processes.
Telomerase allows these specific cells to last in time while remaining functional. Accumulation of telomere malfunctions in these types of cells could trigger tissue and organ degenerescence, one of the main characteristics of aging. In the fight against aging, firm knowledge of the biological mechanisms involving telomerase then seem indispensible.
Telomere shortening: diseases linked to aging
 Telomerase modifications lead to accelerated telomere shortening. This results in the apparition of diseases such as dyskeratosis congenita, which affects tissues that need fast and constant cellular renewal, or like aplastic anemia, that decreases the amount of blood cells [2]. These diseases are often associated with poor tissue and cell renewal, and their symptoms are fairly similar to those of aging.
Telomerase modifications lead to accelerated telomere shortening. This results in the apparition of diseases such as dyskeratosis congenita, which affects tissues that need fast and constant cellular renewal, or like aplastic anemia, that decreases the amount of blood cells [2]. These diseases are often associated with poor tissue and cell renewal, and their symptoms are fairly similar to those of aging.
Telomere length and telomerase therefore seem central to the aging process. This is why it would be interesting to include telomere measurement when developing a metrology of aging.
See all of our articles on “Telomeres and aging”:
Telomeres, at the heart of the aging process
 How and why does telomere shortening seem central to the aging process ? What is their role? How do they influence the aging process? And why do we call telomeres the “biological clocks” of our body?
How and why does telomere shortening seem central to the aging process ? What is their role? How do they influence the aging process? And why do we call telomeres the “biological clocks” of our body?
Part 1: Causes and consequences of telomere shortening during aging
 It is unclear how fast and why telomere shortening speed and aging vary from person to person. Indeed, the possible causes of telomere shortening can vary greatly.
It is unclear how fast and why telomere shortening speed and aging vary from person to person. Indeed, the possible causes of telomere shortening can vary greatly.
Part 2: Accelerated aging due to telomere and telomerase malfunction
 Telomere length and telomerase seem to be key factors of the aging process. Many studies on diseases resulting from mutations on telomerase components have shown that it leads to a lesser quality of cell renewal, which is a phenotype linked to aging.
Telomere length and telomerase seem to be key factors of the aging process. Many studies on diseases resulting from mutations on telomerase components have shown that it leads to a lesser quality of cell renewal, which is a phenotype linked to aging.
Part 3: Telomeres and telomerase in stem cells: central to the aging process
 Telomerase expression diminishes in the few weeks that follow birth in most adult tissues, with the exception of certain types of cells such as stem cells. One can wonder if there is a link between the fact that the quantity of stem cells is lowered with age, telomerase function, and telomere length.
Telomerase expression diminishes in the few weeks that follow birth in most adult tissues, with the exception of certain types of cells such as stem cells. One can wonder if there is a link between the fact that the quantity of stem cells is lowered with age, telomerase function, and telomere length.
Part 4: Towards an aging metrology with telomeres
 To measure aging, several methods based on telomere length have been developed. Today, there are 5 main methods, among which TAT, and STELA. They allow to obtain precious indications on physiological age and aging, from telomere length.
To measure aging, several methods based on telomere length have been developed. Today, there are 5 main methods, among which TAT, and STELA. They allow to obtain precious indications on physiological age and aging, from telomere length.
Part 5: Telomeres and aging, what therapies?
 Telomere length is becoming an interesting lead to elaborate therapies and solutions to fight aging. We can speak of Elizabeth Parrish, CEO of BioViva, a biotech company in the US. She tested on herself two gene therapies developed by her own lab, one of which aims to lengthen her telomeres for “rejuvenation” purposes
Telomere length is becoming an interesting lead to elaborate therapies and solutions to fight aging. We can speak of Elizabeth Parrish, CEO of BioViva, a biotech company in the US. She tested on herself two gene therapies developed by her own lab, one of which aims to lengthen her telomeres for “rejuvenation” purposes
Katidja Allaoui

Author
Auteure
Katidja studied biology and health engineering at the school of engineering of Angers.
More about the Long Long Life team
Katidja a étudié l’ingénierie de la biologie et de la santé à l’école d’ingénieurs de l’université d’Angers.
En savoir plus sur l’équipe de Long Long Life
Dr Guilhem Velvé Casquillas

Author/Reviewer
Auteur/Relecteur
Physics PhD, CEO NBIC Valley, CEO Long Long Life, CEO Elvesys Microfluidic Innovation Center
More about the Long Long Life team
Docteur en physique, CEO NBIC Valley, CEO Long Long Life, CEO Elvesys Microfluidic Innovation Center
En savoir plus sur l’équipe de Long Long Life
Sources :
[1] Chatterjee, S. (2017). Telomeres in health and disease. Journal of Oral and Maxillofacial Pathology, [online] 21(1), p.87. DOI : 10.4103/jomfp.JOMFP_39_16. [Accessed 22 May 2017].
[2] Blasco, M. (2007). Telomere length, stem cells and aging. Nature Chemical Biology, [online] 3(10), pp.640-649. DOI :10.1038/nchembio.2007.38 [Accessed 22 May 2017]
[3] Shay, J. (2016). Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discovery, [online] 6(6), pp.584-593. DOI : 10.1016/j.semcancer.2011.10.001 [Accessed 22 May 2017].