Measuring aging: Telomeres as witnesses of physiological age
Telomere length is a very precise tool to determine the physiological age of our cells and organs.
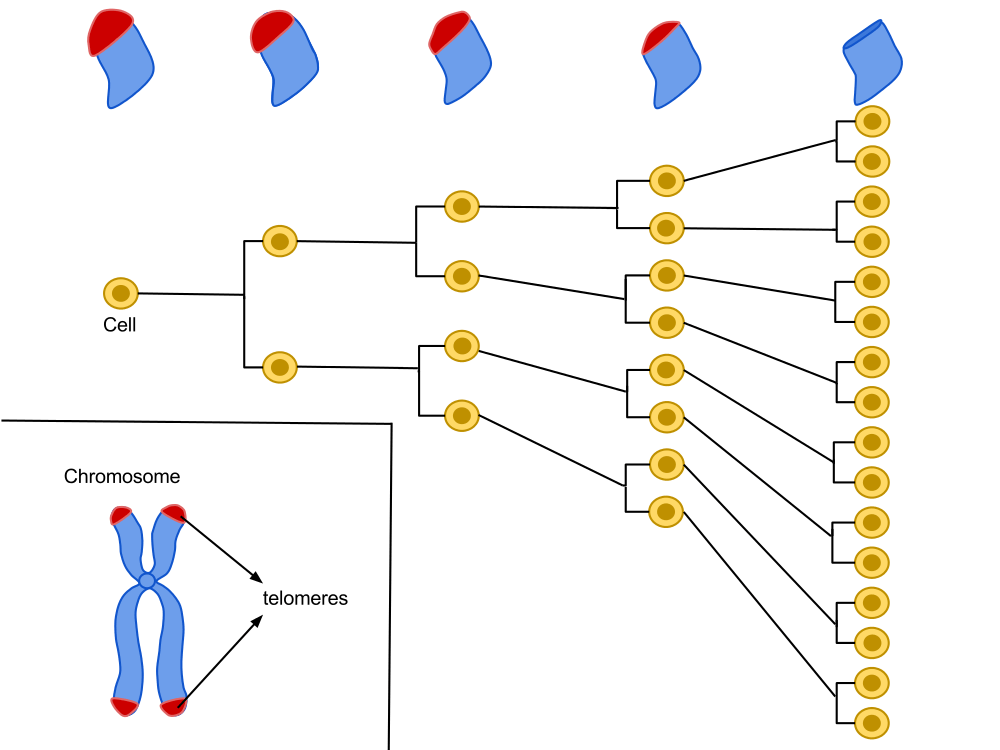
Located at the very end of our chromosomes, telomeres are repetitive sequences of the nucleotide suite TTAGGG. A cell is programed for a given number of divisions. With each division, the ends of the chromosomes become a little shorter, until they reach a critical size. Through numerous reactions, the cell will then stop growing and become senescent.
Lifestyle, diet, stress, environment as well as age can accelerate telomere shortening. Measuring their length can then give us some precious indications on how old our cells really are, as well as what makes them age. For all those reasons, telomere length is a great metrology tool when it comes to measuring the level of aging of an organism, it can influence people into taking control of their health with preventive measures.
Hayflick’s work showed that each cell line has a capital of cellular divisions. This capital is proportional to the longevity of a species and can vary between two individuals of the same species. With each cycle of cell division, chromosome ends (telomeres) lose a fragment of DNA. After several divisions, telomere function, which helps maintain the stability of chromosomes in the DNA, is altered, which could be the basis of our biological clock.
The sample: Telomere length can be measured on cell or tissue samples. However, leucocytes (white blood cells) are the preferred sample for telomere measurement, since they are blood cells and allow for a non-invasive sample collection.
Five main methods to measure telomere length for the metrology of aging
Southern blots of Terminal Restriction Fragments (TRFs)
The most archaic but also the most widely used technique was developed in 1990. It estimates the average number of TTAGGG ending repetitions in the chromosomes.
The technique works by in situ hybridization identifying the TTAGGG repetitions at the end of telomeres. Telomeric DNA is digested by restriction enzymes, which makes for many small DNA segments of different sizes. In order to know the size of each telomeric DNA fragment, the Southern Blot technique allows to separate DNA molecules depending on their length.
The resulting telomeric fragments are separated by gel electrophoresis. In order to make the rest of the process easier, the fragments are transferred onto a nitrocellulose or nylon membrane, which are solid supports that help denature DNA and hybridizing it with a probe.
This is a crucial step for the hybridization of a radioactive probe (a complementary DNA fragment on the given sequence.) The position of the restriction fragments is revealed by radiography and their size is estimated by comparing the distance they traveled in the gel and the distance traveled by fragments of a known length [1, 2].
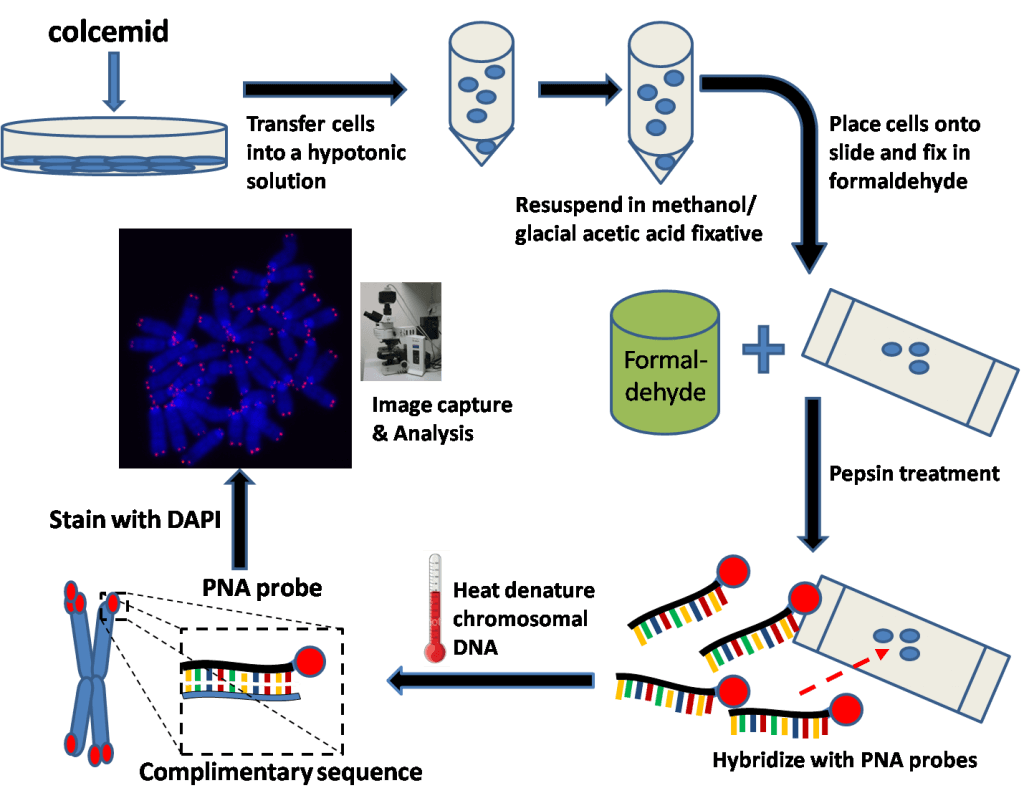
Q-Fish
This method also uses in situ hybridization. However, unlike the Souther Blot method, the probe is not radioactive but fluorescent. The drawback with this method is the important quantity of DNA required (up to 20μg) when only a few ng are sufficient for other PCR-based techniques [3, 4].
Flow-FISH
This technique is similar to the Q-FISH technique in many respects, but Flow-Fisch uses a flow cytometer. This rarely used, expensive technique requires a great deal of equipment. However, it remains very interesting: it allows to reduce the purification steps and to simultaneously analyze telomere length in several cell types [7,8].
TAT to measure telomere length
TAT® estimates the individual length of telomeres. The TAT® technology is based on the high flow, in situ fluorescent hybridization allowed by a High Content Screening (HCS) approach of telomere length analysis. It gives a full measure of telomere length through a histogram. It also gives indications on the shorter and longer telomeres, the frequency by length class, the average length as well as the median values [9].
PCR-Q
Faster and easier, the qPCR technique was developed in 2003. It determines by quantitative PCR the amount of copies of the telomere pattern (T) against a single copy gene (S) with a (T/S) ratio. The number of TTACC repetitions, which are specific to telomeric DNA, is given by the kinetic measure of the polymerization reaction. During the amplification process, the amount of amplicons is monitored with a fluorescent marker which helps to obtain the polymerization reaction kinetic, which in turn helps determine the initial quantity of telomeric DNA [2, 5, 6].
STELA (Single TElomere Length Analysis)
Also based on PCR, this technique shows better resolution than the Southern Blot technique. It measures the length of telomeres by linking a primer on a specific chromosome’s telomere {2, 7, 8].
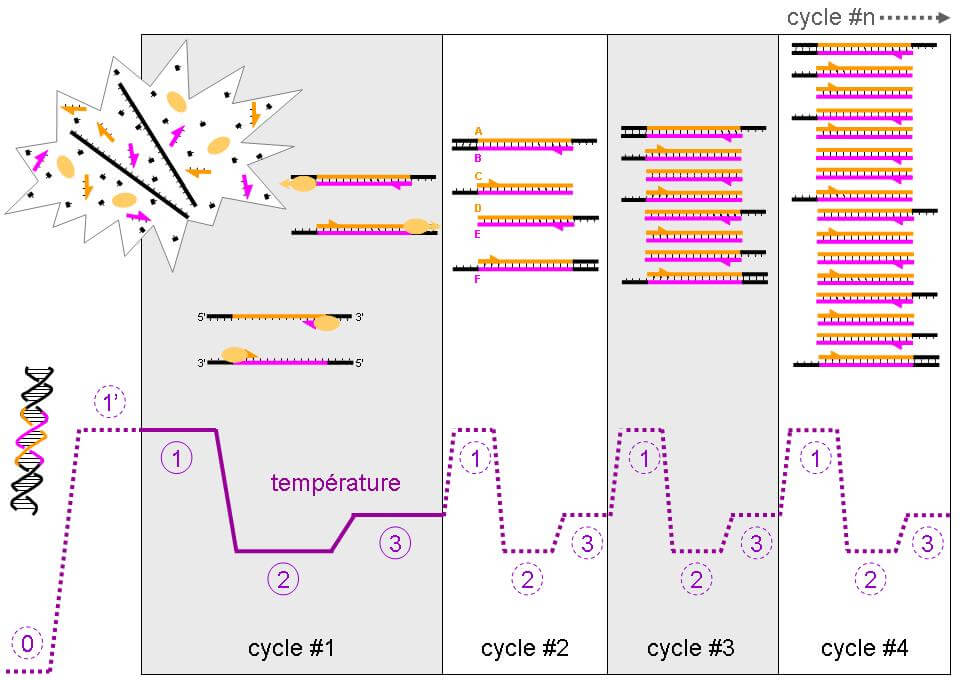
Measuring telomere length and the metrology of aging
PCR techniques might seem better because easily reproducible. However, like other techniques based on in-situ hybridization, they cannot amplify telomeres over 25 kb. It is possible to observe telomeres longer than 50 kb, but the Southern Blot technique is a better tool for this use. We know that not all organs age at the same pace, and even though leucocytes are representative when it comes to studying aging, these tests mostly analyze cell populations from blood samples, which remains simplistic.
Analyzing telomere length from a tissue biopsy is a possibility, but requires specific and exceptional conditions, such as a medical diagnosis. The estimated age is not based on a follow-up in time, but on a comparison to an average in chronological age.
Where can we measure our telomere length?
Many a laboratory has embraced the market, each commercializing its own version of the telomere length analysis kit. The Life Length company is a leader in the field, and own many partner laboratories offering the analysis of samples through the postal service like Les Laboratoires Réunis, or an on-site analysis like La Clinique Crillon.
This futuristic technology is still in its infancy but makes for great future discoveries in the upcoming years, thanks to both the advances in available methods and the mutliplication of laboratories offering such services. They lower the cost, time and risk margin of these analyses.
See all of our articles on “Telomeres and aging”:
Telomeres, at the heart of the aging process
 How and why does telomere shortening seem central to the aging process ? What is their role? How do they influence the aging process? And why do we call telomeres the “biological clocks” of our body?
How and why does telomere shortening seem central to the aging process ? What is their role? How do they influence the aging process? And why do we call telomeres the “biological clocks” of our body?
Part 1: Causes and consequences of telomere shortening during aging
 It is unclear how fast and why telomere shortening speed and aging vary from person to person. Indeed, the possible causes of telomere shortening can vary greatly.
It is unclear how fast and why telomere shortening speed and aging vary from person to person. Indeed, the possible causes of telomere shortening can vary greatly.
Part 2: Accelerated aging due to telomere and telomerase malfunction
 Telomere length and telomerase seem to be key factors of the aging process. Many studies on diseases resulting from mutations on telomerase components have shown that it leads to a lesser quality of cell renewal, which is a phenotype linked to aging.
Telomere length and telomerase seem to be key factors of the aging process. Many studies on diseases resulting from mutations on telomerase components have shown that it leads to a lesser quality of cell renewal, which is a phenotype linked to aging.
Part 3: Telomeres and telomerase in stem cells: central to the aging process
 Telomerase expression diminishes in the few weeks that follow birth in most adult tissues, with the exception of certain types of cells such as stem cells. One can wonder if there is a link between the fact that the quantity of stem cells is lowered with age, telomerase function, and telomere length.
Telomerase expression diminishes in the few weeks that follow birth in most adult tissues, with the exception of certain types of cells such as stem cells. One can wonder if there is a link between the fact that the quantity of stem cells is lowered with age, telomerase function, and telomere length.
Part 4: Towards an aging metrology with telomeres
 To measure aging, several methods based on telomere length have been developed. Today, there are 5 main methods, among which TAT, and STELA. They allow to obtain precious indications on physiological age and aging, from telomere length.
To measure aging, several methods based on telomere length have been developed. Today, there are 5 main methods, among which TAT, and STELA. They allow to obtain precious indications on physiological age and aging, from telomere length.
Part 5: Telomeres and aging, what therapies?
 Telomere length is becoming an interesting lead to elaborate therapies and solutions to fight aging. We can speak of Elizabeth Parrish, CEO of BioViva, a biotech company in the US. She tested on herself two gene therapies developed by her own lab, one of which aims to lengthen her telomeres for “rejuvenation” purposes
Telomere length is becoming an interesting lead to elaborate therapies and solutions to fight aging. We can speak of Elizabeth Parrish, CEO of BioViva, a biotech company in the US. She tested on herself two gene therapies developed by her own lab, one of which aims to lengthen her telomeres for “rejuvenation” purposes
Farah Bahou

Author
Auteure
Farah studied biochemistry, therapeutics and molecular and biopharmaceutical innovation at Aix-Marseille university and Paris 7 Diderot university.
More about the Long Long Life team
Farah a étudié la biochimie, la thérapeutique et les innovations moléculaires et biopharmaceutiques à l’université d’Aix-Marseille et à l’université Paris 7 Diderot.
En savoir plus sur l’équipe de Long Long Life
Dr Guilhem Velvé Casquillas

Author/Reviewer
Auteur/Relecteur
Physics PhD, CEO NBIC Valley, CEO Long Long Life, CEO Elvesys Microfluidic Innovation Center
More about the Long Long Life team
Docteur en physique, CEO NBIC Valley, CEO Long Long Life, CEO Elvesys Microfluidic Innovation Center
En savoir plus sur l’équipe de Long Long Life
Dr. Marion Tible

Author/Reviewer
Auteure/Relectrice
Marion Tible has a PhD in cellular biology and physiopathology. Formerly a researcher in thematics varying from cardiology to neurodegenerative diseases, she is now part of Long Long Life team and is involved in scientific writing and anti-aging research.
More about the Long Long Life team
Marion Tible est docteur en biologie cellulaire et physiopathologie. Ancienne chercheuse dans des thématiques oscillant de la cardiologie aux maladies neurodégénératives, elle est aujourd’hui impliquée au sein de Long Long Life pour la rédaction scientifique et la recherche contre le vieillissement.
En savoir plus sur l’équipe de Long Long Life
References
[1] Kimura, M., Stone, R. C., et al. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nature protocols, 2010, 5(9), 1596-1607.
[2] Martin-Ruiz, C. M., Baird, D., et al. Reproducibility of telomere length assessment: an international collaborative study. International journal of epidemiology, 2014, dyu191.
[3] Cawthon, R. M., Smith, K. R., O’Brien, E., Sivatchenko, A., & Kerber, R. A. Association between telomere length in blood and mortality in people aged 60 years or older. The Lancet, 2003, 361(9355), 393-395.
[4] Lansdorp, P. M., Verwoerd, N. P., et al. Heterogeneity in telomere length of human chromosomes. Human molecular genetics, 1996, 5(5), 685-691.
[5] Gutierrez-Rodrigues, F., Santana-Lemos, B. A., Scheucher, P. S., Alves-Paiva, R. M., & Calado, R. T. Direct comparison of flow-FISH and qPCR as diagnostic tests for telomere length measurement in humans. PLoS One, 2014, 9(11), e113747.
[6] Aviv, A., Hunt, S. C., Lin, J., Cao, X., Kimura, M., & Blackburn, E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic acids research, 2011, gkr634.
[9] Bendix, L., Horn, P. B., Jensen, U. B., Rubelj, I., & Kolvraa, S. The load of short telomeres, estimated by a new method, Universal STELA, correlates with number of senescent cells. Aging cell, 2010, 9(3), 383-397.
[8] Letsolo, B. T., Rowson, J., & Baird, D. M. Fusion of short telomeres in human cells is characterized by extensive deletion and microhomology, and can result in complex rearrangements. Nucleic acids research, 2010, 38(6), 1841-1852.
[9] Baerlocher, G. M., Vulto, I., De Jong, G., & Lansdorp, P. M. Flow cytometry and FISH to measure the average length of telomeres (flow FISH). Nature protocols, 2006, 1(5), 2365-2376.