Why we need motor proteins for anti-aging autophagy
Cytoplasm motor proteins, an essential anti-aging ingredient for autophagy
Autophagy is a cellular process that cleans the cell of defective proteins, lipids and organelles. This phenomenon is part of a set of mechanisms that all participate in proteostasis, i.e. the balance of the entire protein network. Loss of proteostasis is one of nine causes of aging, leading to age-related diseases such as Alzheimer’s disease, Parkinson’s disease or cataracts[1]. Autophagy is effective thanks to two types of cellular compartments, the autophagosomes which trap and store a fragment of cytoplasm to eliminate, and the lysosomes which destroy the contents of the autophagosome. The loss of autophagic capacity with age contributes to the loss of functional capacity of the cells of older organisms[2].
What role do motor proteins play in autophagy?
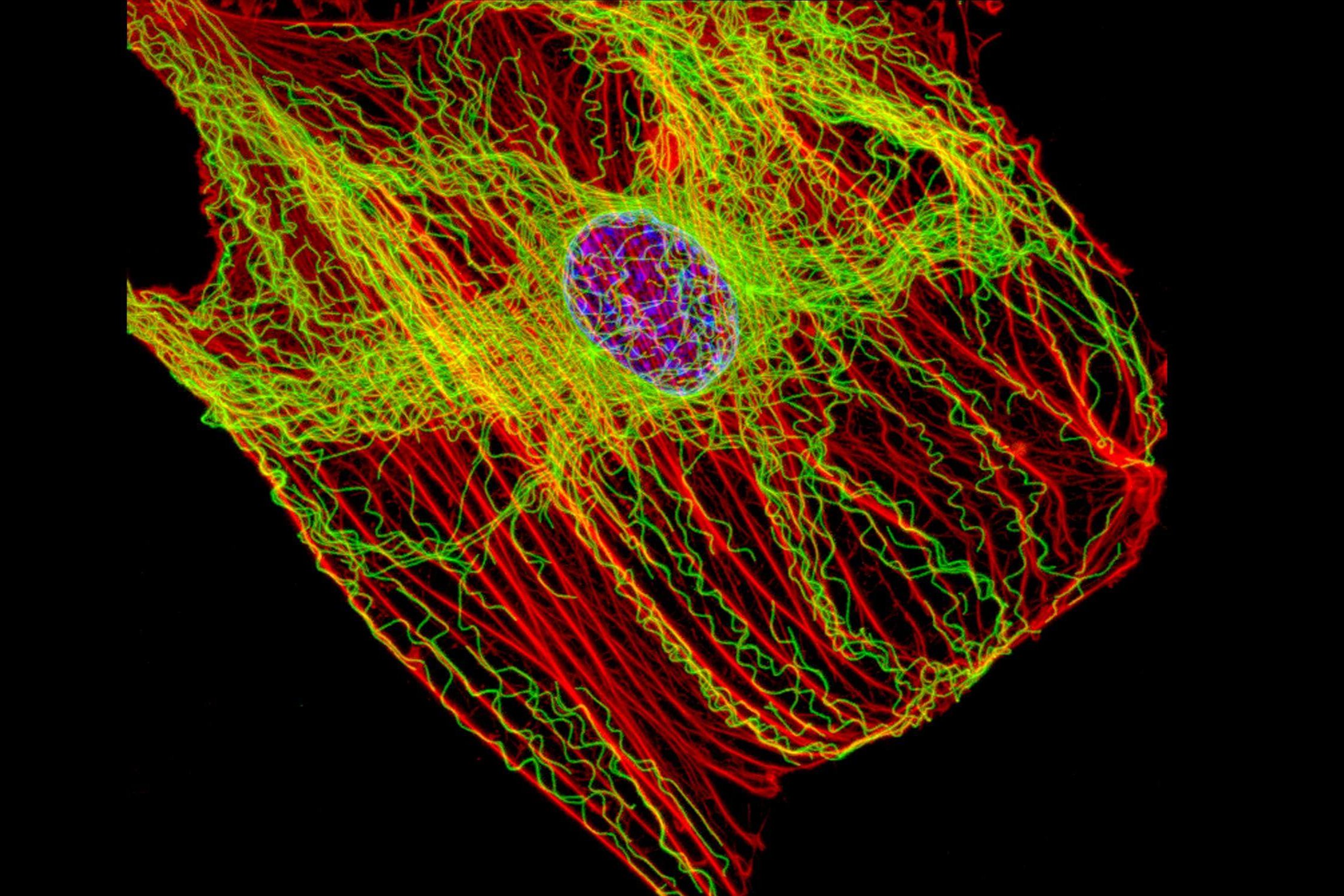
For the lysosomes to degrade the contents of the autophagosomes, the two compartments must merge. This fusion is achieved by their meeting in the cell, in the perinuclear region (near the nucleus) in most cases[2]. In order to move within the cell, the autophagosomes and lysosomes follow the network of microtubules, small tubulin tubes, which allow transport into the cytoplasm. These are small motor proteins, the best known being kinesins and dyneins, which can, thanks to what resembles small molecular feet, move autophagic compartments along microtubules[3]. Positioning autophagosomes and lysosomes correctly in the same cell region is a decisive step in achieving autophagy. The biogenesis of autophagosomes and their degradation by lysosomes decreases with age but the mechanisms underlying this damage are not well known[2].

A Franco-American team of researchers recently published the results of a study[2] to understand the phenomena behind the decline in age-related autophagy. They were particularly interested in the positioning of autophagosomes and lysosomes within the cell, by comparing the movements of these organelles in fibroblasts of young (4 months) and elderly (24 months) mice. The researchers identified differences in the positioning of these compartments between young cells and elderly cells[2]. They then turned their attention to the molecular causes of this difference. Using a genetic approach, scientists mimicked the low amount of KIFC3, a motor protein of the same rank as dyneins and interacting with microtubules, as found in older cells. The results showed that a low level of this protein causes poor positioning of the lysosomes and therefore a decrease in autophagic capacity[2].
Motor proteins as therapeutic anti-aging targets
Autophagy is a mechanism of cell protection against senescence and cell death. When autophagy is effective, it fights aging and has been correlated with longevity in many studies. However, with age, this phenomenon declines, and the molecular mechanisms that are at its origin are still very little characterized. The positioning of autophagosomes and lysosomes near each other is very important to allow their fusion and degradation of defective compounds. The study showed that a bad placement of these entities in the same cellular region is a phenomenon found with age. This defect reduces the effectiveness of the autophagy. The loss of intracellular traffic of autophagous organelles is believed to be due to the decrease in motor protein levels allowing movement of lysosomes and autophagosomes along microtubules. This experiment identified motor proteins as new targets for future therapies that will aim to correct the loss of autophagy in the fight against aging and for longevity.
References :
[1] Carlos Lopez-Otin, Maria A. Blasco, Linda Partridge, Manuel Serrano and Guido Kroemer. The Hallmarks of Aging, Cell 153, June 6, 2013, 1194-1217. http://dx.doi.org/10.1016/j.cell.2013.05.039
[2] Bejarano E, Murray JW, Wang X, et al. Defective recruitment of motor proteins to autophagic compartments contributes to autophagic failure in aging. Aging Cell. 2018;e12777. https://doi.org/10.1111/acel.12777
[3] Anna Akhmanova and Michel O. Steinmetz. Control of microtubule organization and dynamics: two ends in the limelight, NATURE REVIEWS | MOLECULAR CELL BIOLOGY VOLUME 16 | DECEMBER 2015, 711-726.
Anne Fischer

Author
Auteur
Anne is studying medicine science at the Institute of Pharmaceutical and Biological Science in Lyon and she has graduated with a Bachelor’s degree in molecular and cellular biology at the University of Strasbourg.
More about the Long Long Life team
Anne étudie les sciences du médicament à l’Institut des Sciences Pharmaceutiques et Biologiques de Lyon. Elle est titulaire d’une licence en biologie moléculaire et cellulaire de l’Université de Strasbourg.
En savoir plus sur l’équipe de Long Long Life