Telomerase influence on telomeres and aging
Telomerase mutations causing aging acceleration
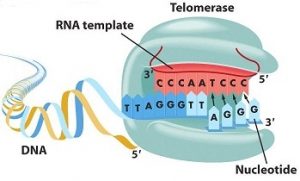
Telomerase is the enzyme in charge of synthesizing telomeres. It allows the TTAGGG repetitions to add up at the end of the chromosomes to reconstitute the telomeres. Telomerase is made of a protein sub-unit, TERT (Telomerase Reverse Transcriptase) which is in charge of telomere synthesis, as well as of an ARN sub-unit, TERC (Telomerase RNA Component) used as a synthesis model [1].
Many studies have been conducted on mice devoid of the gene that codes the TERC sub-unit. The goal was to determine the consequences of telomere shortening on the aging process and on cancer occurrence. It was discovered that without the gene, the long-term viability of the mice was severely compromised [2]. The surviving group of mice showed many symptoms linked to the loss of telomeric repetitions: decrease fertility, heart failure, immuno-senescence (progressive deterioration of the immune system, triggered by aging), decreased gut, skin and red blood cells renewal, etc [2]. The symptoms usually appear with age and confirm the existence of a link between aging and telomerase malfunction.
Telomeres and telomerase seem to impact the aging process Any mutation affecting telomerase components was shown to trigger dysketarosis congenita, aplastic anemia, myelodysplastic syndromes and leukemia [1]. Poor cell renewal and severe tissue degeneration are two symptoms common to all of these diseases, and they are sign generally linked to aging [1]. Although the precise mechanisms involved are not yet clear, telomerase seems to play an important role in the aging process and our longevity.
Many aging-induced diseases also linked to mutations of the genes coding telomerase
The study of human diseases linked to mutations of the genes coding for telomerase led to the discovery of the limiting role of telomeres on longevity, and of the impact of telomerase on aging.
In a previous article (Telomeres, at the heart of the aging process) we mentioned that telomere length might help predict the occurrence of replicative senescence, which is when the life cycle of the cell comes to a stop. Telomere alteration is then linked to a decrease of cellular metabolism. The accumulation of such cells in an organism triggers the expression of age-linked phenotypes in the form of several diseases [1].
Cardiovascular diseases
Heart failure is among the leading causes of death in elderly people. As a result, researchers have been studying the link between telomere shortening, which accelerates in the old age, and the development of heart failure. They analyzed heart function in knock-out mice, whose coding gene for telomerase was silenced in their embryonic stem cells. In many generations of these TERC(-/-) mice, there was a significant decrease of life expectancy due to telomere shortening, which would be linked to a decrease in cell proliferation, an increase in apoptosis and hypertrophia (when the muscle cells in the heart grow too much) [3]. In response to these effects, ventricular dilatation, thinner vascular walls and heart failure were observed [3].
Faster telomere shortening due to malfunctioning telomerase would also be linked to aging-linked diseases such as cardiovascular diseases. Furthermore, other studies have shown a link between telomere shortening and premature death due to cardiovascular diseases or infection [4]. If aging in humans triggers issues with telomerase regulation, this knowledge could lead us to innovative preventive therapies against cardiovascular diseases, in order to prolong healthspan.
Dementia and cognitive disorders

Researchers showed a correlation between telomere shortening and the occurrence of mental disorders: higher perceived stress levels, cognitive impairments and depressive states [5]. In patients with telomere-induced accelerated telomere shortening, there were cases of schizophrenia and mood disorders [6]. These conditions, which frequently develop in old age, can help us confirm the key role of telomerase and telomeres in the aging process.
Poor cellular renewal and tissue degradation
Among the conditions linked to TERC mutations, we find some causing poor cellular renewal. Dyskeratosis congenita, also called Zinsser-Cole-Engman syndrome, is one of them. It is characterized by fast telomere attrition. The most severe cases include serious clinical signs, such as: medullary aplasia (bone marrow attrition), neutropenia ( lower white blood cells count), thrombopenia (lower platelets count) pulmonary fibrosis, global immune deficiency and cancer. Today, there is no treatment for the disease, except for hematopoietic stem cells transplant which seems to have positive results [2].
Mutated telomerase-coding genes play a role in the degradation of the organism at every level: poor cellular renewal, repair and protection systems malfunction, tissue degenerescence, etc [2]. There are many diseases linked to it. Aplastic anemia is characterized by a low red blood cells count, and idiopathic pulmonary fibrosis, which is deadly, is characterized by lung scarring that brings on respiratory difficulty.
The origin of telomerase malfunction and accelerated aging
Telomeres then become indispensible for maintaining the cell’s life cycle. However, even though an imbalance in the enzymatic activity of telomerase can accelerate aging, a surexpression of the enzyme could induce excessive cell proliferation and potentially increase the risk of developing tumors [2]. Therefore, regarding future therapies, it would be interesting to look into targeted treatments. A telomere therapy targeting solely stem cells could solve age-related cellular renewal issues without inducing cancer in the rest of the organism (cf Part 3).
See all of our articles on “Telomeres and aging”:
Telomeres, at the heart of the aging process
 How and why does telomere shortening seem central to the aging process ? What is their role? How do they influence the aging process? And why do we call telomeres the “biological clocks” of our body?
How and why does telomere shortening seem central to the aging process ? What is their role? How do they influence the aging process? And why do we call telomeres the “biological clocks” of our body?
Part 1: Causes and consequences of telomere shortening during aging
 It is unclear how fast and why telomere shortening speed and aging vary from person to person. Indeed, the possible causes of telomere shortening can vary greatly.
It is unclear how fast and why telomere shortening speed and aging vary from person to person. Indeed, the possible causes of telomere shortening can vary greatly.
Part 2: Accelerated aging due to telomere and telomerase malfunction
 Telomere length and telomerase seem to be key factors of the aging process. Many studies on diseases resulting from mutations on telomerase components have shown that it leads to a lesser quality of cell renewal, which is a phenotype linked to aging.
Telomere length and telomerase seem to be key factors of the aging process. Many studies on diseases resulting from mutations on telomerase components have shown that it leads to a lesser quality of cell renewal, which is a phenotype linked to aging.
Part 3: Telomeres and telomerase in stem cells: central to the aging process
 Telomerase expression diminishes in the few weeks that follow birth in most adult tissues, with the exception of certain types of cells such as stem cells. One can wonder if there is a link between the fact that the quantity of stem cells is lowered with age, telomerase function, and telomere length.
Telomerase expression diminishes in the few weeks that follow birth in most adult tissues, with the exception of certain types of cells such as stem cells. One can wonder if there is a link between the fact that the quantity of stem cells is lowered with age, telomerase function, and telomere length.
Part 4: Towards an aging metrology with telomeres
 To measure aging, several methods based on telomere length have been developed. Today, there are 5 main methods, among which TAT, and STELA. They allow to obtain precious indications on physiological age and aging, from telomere length.
To measure aging, several methods based on telomere length have been developed. Today, there are 5 main methods, among which TAT, and STELA. They allow to obtain precious indications on physiological age and aging, from telomere length.
Part 5: Telomeres and aging, what therapies?
 Telomere length is becoming an interesting lead to elaborate therapies and solutions to fight aging. We can speak of Elizabeth Parrish, CEO of BioViva, a biotech company in the US. She tested on herself two gene therapies developed by her own lab, one of which aims to lengthen her telomeres for “rejuvenation” purposes
Telomere length is becoming an interesting lead to elaborate therapies and solutions to fight aging. We can speak of Elizabeth Parrish, CEO of BioViva, a biotech company in the US. She tested on herself two gene therapies developed by her own lab, one of which aims to lengthen her telomeres for “rejuvenation” purposes
Katidja Allaoui

Author
Auteure
Katidja studied biology and health engineering at the school of engineering of Angers.
More about the Long Long Life team
Katidja a étudié l’ingénierie de la biologie et de la santé à l’école d’ingénieurs de l’université d’Angers.
En savoir plus sur l’équipe de Long Long Life
Dr Guilhem Velvé Casquillas

Author/Reviewer
Auteur/Relecteur
Physics PhD, CEO NBIC Valley, CEO Long Long Life, CEO Elvesys Microfluidic Innovation Center
More about the Long Long Life team
Docteur en physique, CEO NBIC Valley, CEO Long Long Life, CEO Elvesys Microfluidic Innovation Center
En savoir plus sur l’équipe de Long Long Life
Sources :
[1] Chatterjee, S. (2017). Telomeres in health and disease. Journal of oral and maxillofacial pathology: JOMFP, 21(1), 87.
[2] Blasco, M. A. (2007). Telomere length, stem cells and aging. Nature chemical biology, 3(10), 640-649.
[3] Leri, A., Franco, S., Zacheo, A., Barlucchi, L., Chimenti, S., Limana, F., … & Blasco, M. A. (2003). Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. The EMBO journal, 22(1), 131-139.
[4] Cawthon, R. M., Smith, K. R., O’Brien, E., Sivatchenko, A., & Kerber, R. A. (2003). Association between telomere length in blood and mortality in people aged 60 years or older. The Lancet, 361(9355), 393-395.
[5] Canela, A., Vera, E., Klatt, P. & Blasco, M.A. High-thoughput telomere length quantification by FISH and its application to human population studies. Proc. Natl. Acad. Sci. USA 104, 5300–5305 (2007).
[6] Teyssier, J. R., Ragot, S., Donzel, A., & Chauvet-Gelinier, J. C. (2010). Longueur des télomères dans le cortex des patients atteints de troubles dépressifs. L’Encéphale, 36(6), 491-494.
*https://planet-vie.ens.fr/article/1813/invalidation-gene-knock-out

![[Video] Eurosymposium on Healthy Ageing, Brussels, 2018 Eurosymposium on Healthy Aging](http://www.longlonglife.org/wp-content/uploads/2019/07/P1310252-218x150.jpg)